Features
-
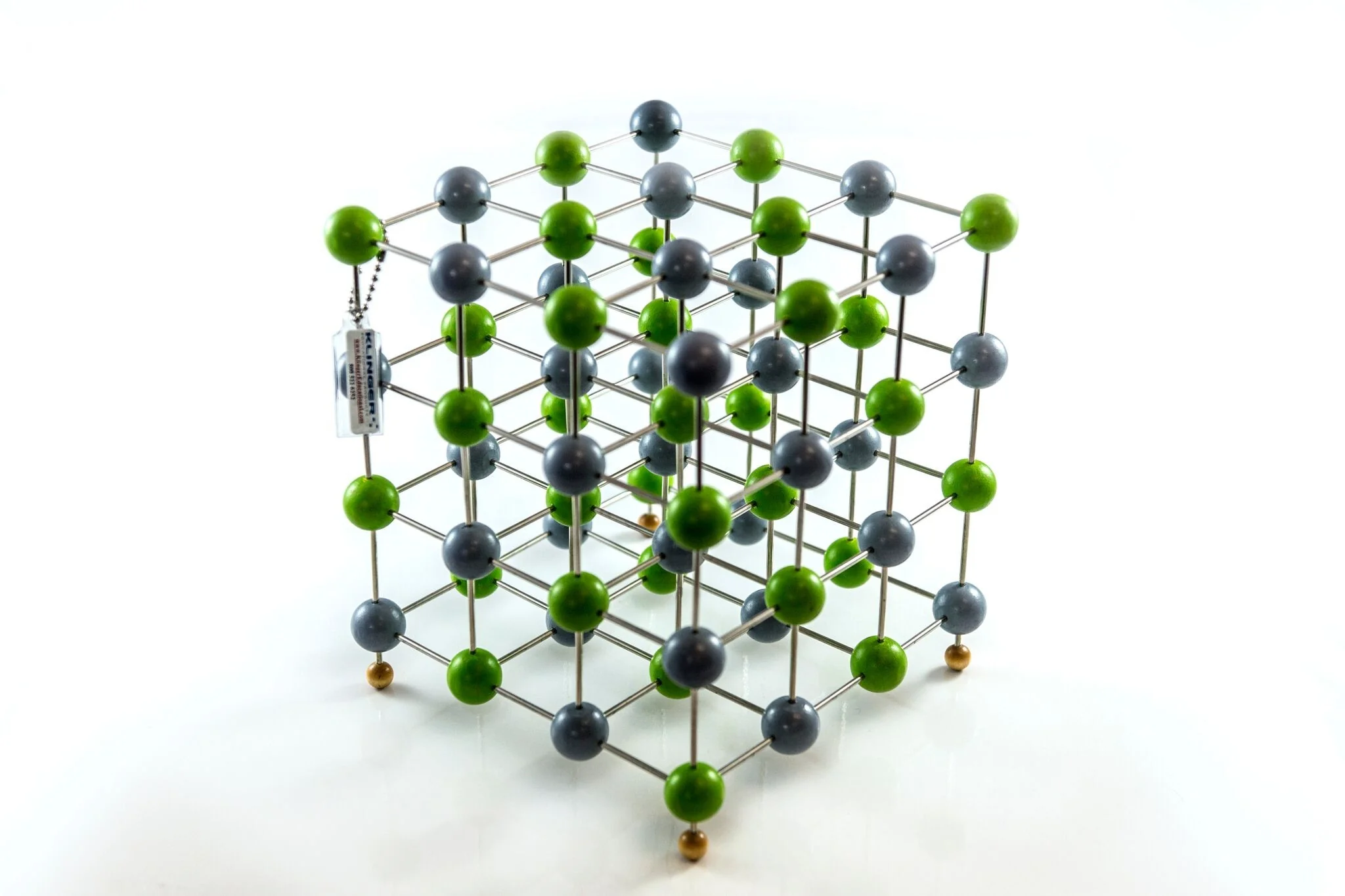
Cubic Structure: Ions are arranged in a perfect repeating cube (face-centered cubic lattice).
-
Ionic Bonding: Strong electrostatic attraction between Na⁺ and Cl⁻ ions.
-
1:1 Ratio: Equal number of sodium and chloride ions throughout the crystal.
-
High Stability: The structure is very stable, giving salt its high melting and boiling points.
-
Coordination Number: Each Na⁺ ion is surrounded by 6 Cl⁻ ions, and vice versa (6:6 coordination).
-
Hard and Brittle: The rigid lattice makes it hard but easily fractured under pressure.
-
Transparent Appearance: Pure NaCl crystals are clear and colorless.


There are no reviews yet.